________________
87. A.K. Shaha, Combustion Engineering and Fuel Technology, p. 28
“Fuels may be classified in various ways, i.e., 1. According to the physical
state in which they exist in nature-solid, liquid and gaseous ...." 88. A.K. Shaha, Combustion Engineering and Fuel Technology, p. 128–
“Chemical reactions among substances take place more quickly and intensively, the closer is the contact among the reacting substances and the larger the surface of contact. The duration of a chemical reaction depends upon thermal conditions, which might be created either before the beginning of reaction, or during the reaction. All chemical reactions, in the present case combustion, i.e. , combination of substances with oxygen, are based on the above mentioned principles. Combustion or combination of substances with either pure oxygen or oxygen contained in the air is realised in practice with the help of different contrivances,
the so-called burners." 89. A.K. Shaha, Combustion Engineering and Fuel Technology, p. 129
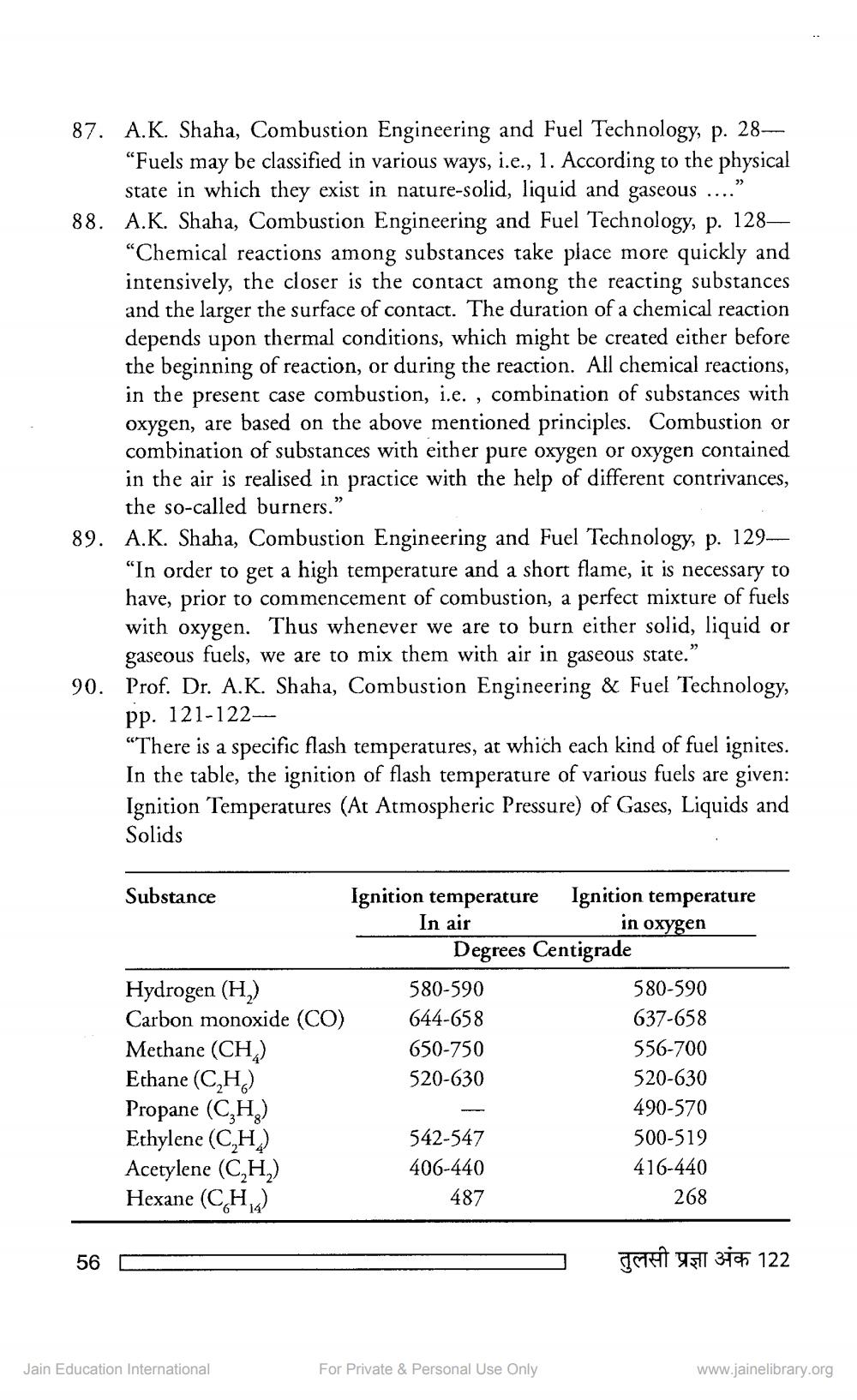
“In order to get a high temperature and a short flame, it is necessary to have, prior to commencement of combustion, a perfect mixture of fuels with oxygen. Thus whenever we are to burn either solid, liquid or gaseous fuels, we are to mix them with air in gaseous state.” Prof. Dr. A.K. Shaha, Combustion Engineering & Fuel Technology, pp. 121-122"There is a specific flash temperatures, at which each kind of fuel ignites. In the table, the ignition of flash temperature of various fuels are given: Ignition Temperatures (At Atmospheric Pressure) of Gases, Liquids and Solids
90
Substance
Ignition temperature Ignition temperature In air
in oxygen
Degrees Centigrade Hydrogen (H2)
580-590
580-590 Carbon monoxide (CO) 644-658
637-658 Methane (CH)
650-750
556-700 Ethane ( CH) 520-630
520-630 Propane (CH)
490-570 Ethylene (CH)
542-547
500-519 Acetylene (C,H,)
406-440
416-440 Hexane (CH2)
487
268
56 D
]
IMG 4311 310 122
Jain Education International
For Private & Personal Use Only
www.jainelibrary.org